Principal Quantum Number: Definition, Importance, and Applications
The Principal Quantum Number (n) is a fundamental concept in quantum mechanics that describes the energy levels of electrons in an atom. It plays a crucial role in determining the size, energy, and position of an electron within an atom. Understanding the Principal Quantum Number is essential for grasping atomic structure, electron configurations, and the behavior of elements in the periodic table.
In this article, we will explore the definition, significance, rules, and real-life applications of the Principal Quantum Number (n) in atomic theory.
What is the Principal Quantum Number?
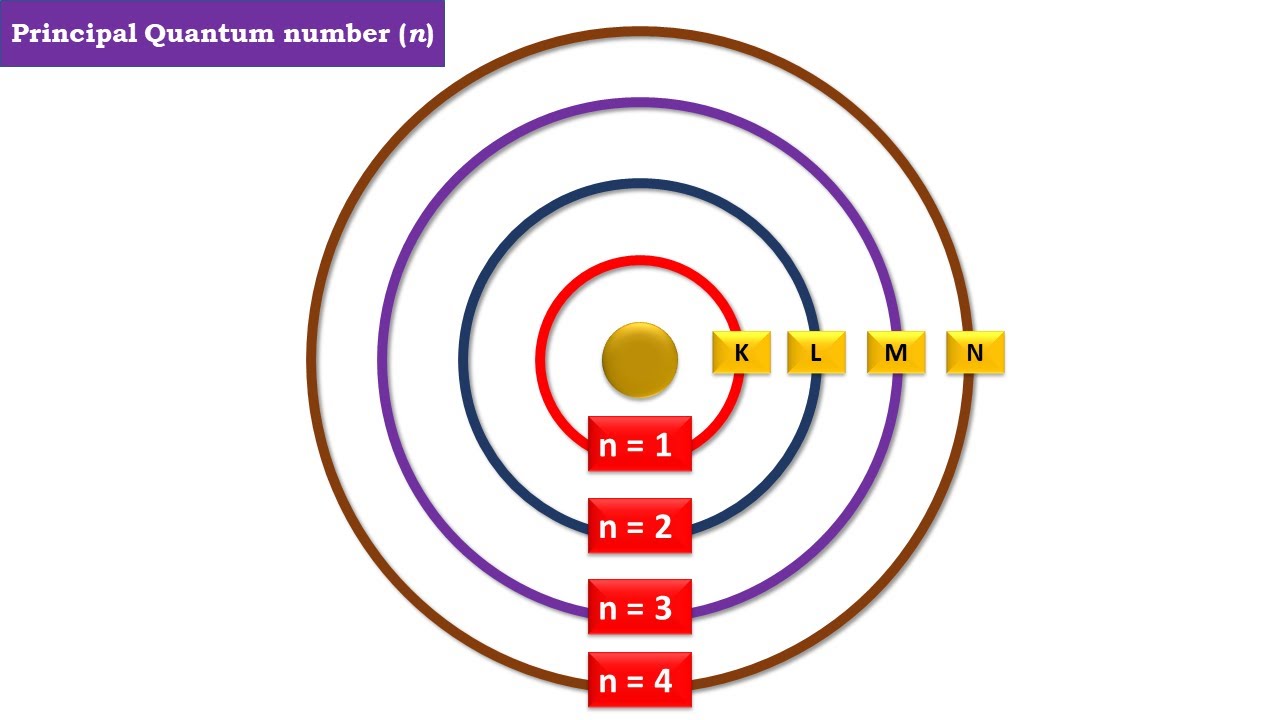
The Principal Quantum Number (n) is a positive integer (n = 1, 2, 3, …) that represents the main energy level or shell of an electron in an atom. It determines:
- The energy of the electron
- The distance of the electron from the nucleus
- The maximum number of electrons in each shell
Each energy level corresponds to a shell (K, L, M, N, etc.), with n = 1 being the lowest energy level and higher values of n representing higher energy levels farther from the nucleus.
Significance of the Principal Quantum Number (n)
Determines Energy Levels
- The value of n specifies the energy level where the electron is located.
- Higher n values indicate electrons in higher energy states and farther from the nucleus.
Defines Atomic Size
- A higher n means the electron is further from the nucleus, leading to a larger atomic size.
Determines Electron Capacity in Shells
- The maximum number of electrons in each shell is given by the formula:
Maximum Electrons = 2n²
- The maximum number of electrons in each shell is given by the formula:
Influences Chemical Reactivity
- The outermost energy level (valence shell) defines an element’s reactivity and bonding behavior.
Values of Principal Quantum Number and Electron Capacity
| Shell | Principal Quantum Number (n) | Maximum Electrons (2n² Rule) |
|---|---|---|
| K-Shell | 1 | 2 |
| L-Shell | 2 | 8 |
| M-Shell | 3 | 18 |
| N-Shell | 4 | 32 |
| O-Shell | 5 | 50 |
As the value of n increases, the electron capacity also increases, allowing more electrons to occupy higher shells.
Rules Associated with the Principal Quantum Number
The Principal Quantum Number follows certain rules that define how electrons are arranged in an atom:
1. The Aufbau Principle
- Electrons fill lower energy levels first before occupying higher ones.
- Example: The 1s orbital (n=1) fills before 2s (n=2).
2. The Pauli Exclusion Principle
- No two electrons in an atom can have the same set of four quantum numbers.
- The Principal Quantum Number (n) is one of the four quantum numbers.
3. Hund’s Rule
- Electrons occupy orbitals singly before pairing up within the same energy level.
These principles help define electron configurations in atoms, which influence their chemical and physical properties.
Principal Quantum Number and the Periodic Table
The Periodic Table is structured based on energy levels (n values):
- Elements in Period 1 (n = 1) – Hydrogen (H) and Helium (He).
- Elements in Period 2 (n = 2) – Lithium (Li) to Neon (Ne).
- Elements in Period 3 (n = 3) – Sodium (Na) to Argon (Ar).
Each period (row) in the periodic table corresponds to a Principal Quantum Number (n), indicating the valence shell of electrons for those elements.
Real-Life Applications of Principal Quantum Number
The concept of Principal Quantum Number (n) is widely used in various scientific and technological fields:
1. Spectroscopy and Atomic Emission Spectra
- When electrons jump between energy levels, they emit or absorb energy in the form of light.
- The emitted light helps in studying elemental composition in stars, planets, and laboratory research.
2. Quantum Mechanics and Wave Functions
- The wave nature of electrons is described using quantum numbers, including n.
- Used in computational chemistry, nanotechnology, and semiconductor physics.
3. X-Ray Production and Medical Imaging
- X-rays are produced when electrons transition between inner shells (like K-shell to L-shell).
- Applied in X-ray machines and medical diagnostics.
4. Laser Technology
- The concept of electron energy levels is used in laser light production.
- Helps in fiber optics, communication, and industrial applications.
5. Semiconductor Physics and Electronics
- Quantum mechanics, including Principal Quantum Numbers, is essential for understanding semiconductors, transistors, and microchips.
Difference Between Principal Quantum Number and Other Quantum Numbers
| Quantum Number | Symbol | Defines |
|---|---|---|
| Principal Quantum Number | n | Energy level (shell) |
| Azimuthal Quantum Number | l | Subshell (s, p, d, f) |
| Magnetic Quantum Number | mₗ | Orbital orientation |
| Spin Quantum Number | mₛ | Electron spin (+½ or -½) |
While n determines the energy level, other quantum numbers define subshells, orbitals, and spin behavior.
Frequently Asked Questions (FAQs)
1. Can the Principal Quantum Number be zero?
No, the Principal Quantum Number (n) must be a positive integer (1, 2, 3, …). It cannot be zero.
2. What is the maximum number of electrons in n = 4?
Using the formula 2n², for n = 4:
Maximum electrons = 2 × (4²) = 32 electrons.
3. How does the Principal Quantum Number affect atomic size?
As n increases, the electron shells are farther from the nucleus, leading to a larger atomic radius.
4. Why do elements in the same period have the same Principal Quantum Number?
Elements in the same period have electrons in the same outer energy level (same n value), which influences their chemical properties.
5. How does the Principal Quantum Number relate to electron configuration?
Electron configuration follows the sequence of increasing n values, determining how electrons are arranged in an atom.
Conclusion
The Principal Quantum Number (n) is a crucial concept in atomic structure and quantum mechanics. It determines the energy level, size, and electron capacity of an atom, influencing chemical properties and periodic trends.
From spectroscopy to semiconductor technology, understanding the Principal Quantum Number is essential for advancements in physics, chemistry, and modern electronics.