Magnetic Quantum Number Explained in Short
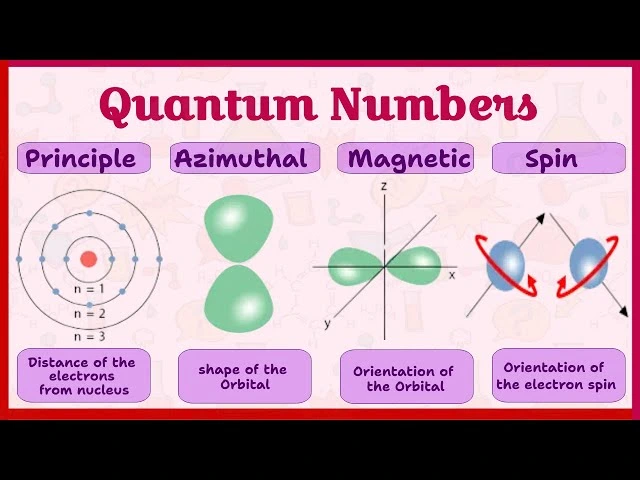
The magnetic quantum number is a crucial concept in quantum mechanics, helping us understand the spatial orientation of atomic orbitals. It plays a significant role in determining the arrangement of electrons around an atom’s nucleus and explains the behavior of electrons in magnetic fields. This article delves into the definition, importance, and applications of the magnetic quantum number in chemistry and physics.
What is the Magnetic Quantum Number?
The magnetic quantum number, denoted by m, is one of the four quantum numbers that describe the unique quantum state of an electron in an atom. It specifies the orientation of an atomic orbital in a magnetic field, relative to the other orbitals in the same subshell.
Key Features:
- Dependent on the Azimuthal Quantum Number (l):
- The magnetic quantum number ranges from -l to +l, including zero.
- For example, if (d orbital), then .
- Defines Orbital Orientation:
- While the azimuthal quantum number determines the shape of an orbital (s, p, d, f), the magnetic quantum number specifies how these orbitals are oriented in three-dimensional space.
- Integral Value:
- The magnetic quantum number can only have integer values, depending on the value.
The Relationship Between Quantum Numbers
To understand the magnetic quantum number, it’s essential to see how it fits within the broader framework of quantum numbers:
- Principal Quantum Number (n):
- Indicates the energy level or shell of an electron.
- Values: 1, 2, 3, …
- Azimuthal Quantum Number (l):
- Defines the subshell (s, p, d, f) and the shape of the orbital.
- Values: 0 to .
- Magnetic Quantum Number (m):
- Specifies the orientation of the orbital.
- Values: to .
- Spin Quantum Number (m):
- Represents the spin of the electron.
- Values: or .
How the Magnetic Quantum Number Works
Each subshell (s, p, d, f) consists of orbitals with specific orientations, determined by the magnetic quantum number:
- s Subshell (l = 0):
- Only one orbital; .
- p Subshell (l = 1):
- Three orbitals; .
- d Subshell (l = 2):
- Five orbitals; .
- f Subshell (l = 3):
- Seven orbitals; .
Each orbital defined by a unique magnetic quantum number can hold a maximum of two electrons with opposite spins.
Significance of the Magnetic Quantum Number
1. Orbital Orientation
- The magnetic quantum number determines the spatial arrangement of orbitals around the nucleus. This information is vital for understanding molecular geometry and bonding.
2. Spectral Lines
- In the presence of a magnetic field, the splitting of spectral lines (known as the Zeeman effect) is explained by the magnetic quantum number.
3. Electron Distribution
- Helps describe how electrons are distributed in an atom, which is crucial for predicting chemical properties.
4. Chemical Bonding
- The orientation of orbitals influences the formation of covalent bonds and hybridization in molecules.
Applications of the Magnetic Quantum Number
1. Atomic Structure
- The magnetic quantum number is fundamental to understanding atomic models and electron configurations.
2. Molecular Bonding
- Used to predict orbital overlap and bonding patterns in molecules, essential for valence bond theory and molecular orbital theory.
3. Spectroscopy
- Explains how atoms and molecules interact with electromagnetic radiation, aiding in the study of spectra.
4. Magnetism
- Provides insight into how electrons behave in magnetic fields, contributing to the development of materials like ferromagnets and paramagnets.
5. Quantum Chemistry
- Integral to solving the Schrödinger equation for multi-electron systems, helping scientists understand the quantum behavior of atoms and molecules.
Examples of Magnetic Quantum Number Values
- For (p orbital):
- Possible : -1, 0, +1.
- Indicates three p orbitals oriented along the x, y, and z axes.
- For (d orbital):
- Possible : -2, -1, 0, +1, +2.
- Specifies five d orbitals with unique spatial orientations.
- For (f orbital):
- Possible : -3, -2, -1, 0, +1, +2, +3.
- Represents seven f orbitals with distinct directions.
Differences Between Azimuthal and Magnetic Quantum Numbers
| Aspect | Azimuthal Quantum Number (l) | Magnetic Quantum Number (m) |
|---|---|---|
| Purpose | Defines the shape of orbitals (s, p, d, f). | Specifies the orientation of orbitals in space. |
| Values | 0 to . | -l to +l, including 0. |
| Dependence | Dependent on the principal quantum number (n). | Dependent on the azimuthal quantum number (l). |
Conclusion
The magnetic quantum number (m) is a vital quantum mechanical concept that helps describe the spatial orientation of orbitals in an atom. By determining how orbitals are arranged in a magnetic field, it provides critical insights into atomic structure, electron behavior, and chemical bonding.